Difference between revisions of "Adalimumab"
Techsensus (talk | contribs) |
Techsensus (talk | contribs) |
||
| Line 164: | Line 164: | ||
|2 hours | |2 hours | ||
|- | |- | ||
| − | |R-Biopharm AG | + | |R-Biopharm AG<ref name=”[30]”>GN3043 RIDA®QUICK ADM Monitoring (2018). Leaflet ''GN3043 RIDA®QUICK ADM Monitoring''. R-Biopharm AG.</ref> |
|GN3043 | |GN3043 | ||
|RIDA®QUICK ADM Monitoring | |RIDA®QUICK ADM Monitoring | ||
| Line 173: | Line 173: | ||
|15 min. | |15 min. | ||
|- | |- | ||
| − | | | + | |BÜHLMANN<ref name=”[31]”>LF-TLAD25 Quantum Blue® Adalimumab (2018). Leaflet ''LF-TLAD25 Quantum Blue® Adalimumab''. BÜHLMANN.</ref> |
|LF-TLAD25 | |LF-TLAD25 | ||
|Quantum Blue® Adalimumab | |Quantum Blue® Adalimumab | ||
| Line 190: | Line 190: | ||
== Numbers == | == Numbers == | ||
| − | Rheumatoid arthritis affects around 1% of the world population per year.<ref name=”[ | + | Rheumatoid arthritis affects around 1% of the world population per year.<ref name=”[32]”>Marita Cross, M. et al. (2014). The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. ''Annals of Rheumatic diseases, 73'', 1316-1322. [https://doi.org/10.1136/annrheumdis-2013-204627 ''doi:10.1136/annrheumdis-2013-204627'']</ref> Global estimates in 2010 reported a prevalence rate of 0.35% for women and 0.13% for men. The prevalence of RA is higher in more developed countries.<ref name=”[33]”>Fazal, S.A. et al. (2018). A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. ''Endocrine, Metabolic & Immune disorders - Drug Targets, 18''(2), 98-109. [https://doi.org/10.2174/1871530317666171114122417 ''doi:10.2174/1871530317666171114122417'']</ref> A study in the US reported an overall lifetime risk for RA of 3.6% for women and 1.7% for men. This corresponds to around 1 in 28 women and 1 in 59 men that will develop RA in their lifetime.<ref name=”[34]”>Crowson, C.S. et al. (2011). The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. ''Arthritis and Rheumatism, 63''(3), 633-639. [https://doi.org/10.1002/art.30155 ''doi:10.1002/art.30155'']</ref> |
| − | Globally in 2010, RA represented 0.49% of years lived with disability (YLD) and 0.19% of disability-adjusted-years (DALY). Across 31 nations in the period of 2009–2011, a total of 219,189 patients died, in whom RA was registered as the underlying cause of death. The YLDs for RA were 55/100000 population and the total DALYs were around 4.8 million.<ref name=”[ | + | Globally in 2010, RA represented 0.49% of years lived with disability (YLD) and 0.19% of disability-adjusted-years (DALY). Across 31 nations in the period of 2009–2011, a total of 219,189 patients died, in whom RA was registered as the underlying cause of death. The YLDs for RA were 55/100000 population and the total DALYs were around 4.8 million.<ref name=”[33]”>Fazal, S.A. et al. (2018). A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. ''Endocrine, Metabolic & Immune disorders - Drug Targets, 18''(2), 98-109. [https://doi.org/10.2174/1871530317666171114122417 ''doi:10.2174/1871530317666171114122417'']</ref> |
| − | The US reported having approximately $128 billion of direct and $47.0 billion of indirect costs billable to arthritis and related rheumatic conditions. In the UK, this number came down to £560 million a year in health care costs.<ref name=”[ | + | The US reported having approximately $128 billion of direct and $47.0 billion of indirect costs billable to arthritis and related rheumatic conditions. In the UK, this number came down to £560 million a year in health care costs.<ref name=”[33]”>Fazal, S.A. et al. (2018). A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. ''Endocrine, Metabolic & Immune disorders - Drug Targets, 18''(2), 98-109. [https://doi.org/10.2174/1871530317666171114122417 ''doi:10.2174/1871530317666171114122417'']</ref> |
| − | In 2017, adalimumab (Humira) was at the top of pharmaceutical products by sales worldwide. The drug generated more than 18.4 billion US dollars. Almost twice as much as Rituxan, who took second place with 9.2 billion dollars generated.<ref name=”[ | + | In 2017, adalimumab (Humira) was at the top of pharmaceutical products by sales worldwide. The drug generated more than 18.4 billion US dollars. Almost twice as much as Rituxan, who took second place with 9.2 billion dollars generated.<ref name=”[35]”>Statista (2018). Top 15 pharmaceutical products by sales worldwide in 2017. Accessed on 7 November 2018, at [https://www.statista.com/statistics/258022/top-10-pharmaceutical-products-by-global-sales-2011/ ''https://www.statista.com/statistics/258022/top-10-pharmaceutical-products-by-global-sales-2011/''].</ref> In 2015, Humira costs around $2669 per month in the US and $1362 in the UK.<ref name=”[36]”>Statista (2018). Average prices of Humira in selected countries in 2015. Accessed on 7 November 2018, at [https://www.statista.com/statistics/312014/average-price-of-humira-by-country/ ''https://www.statista.com/statistics/312014/average-price-of-humira-by-country/''].</ref> |
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 15:17, 4 February 2019
 [1] [1]
| |
| Drug Name | Adalimumab |
|---|---|
| Systematic name | Immunoglobulin G1, anti-(human tumor necrosis factor) (human monoclonal D2E7 heavy chain), disulfide with human monoclonal D2E7 light chain, dimer[2] |
| Synonyms | ADL, D2E7, adalimumab-adaz, adalimumab-adbm, adalimumab-atto[1] |
| Type | Monoclonal antibody (mAb), Biologics, Disease-modifying antirheumatic drugs (DMARD)[1] |
| Target | Tumor necrosis factor alpha (TNF-⍺) |
| Molecular formula | C6428H9912N1694O1987S46[1] |
| Molecular weight | 148 kDa[1] |
| Half life | ~20 days[3] |
Contents
Structure and History
Structure
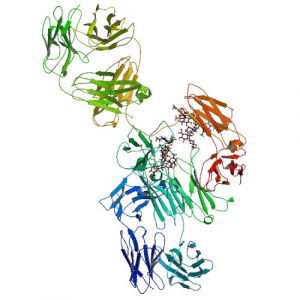
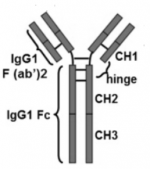
Adalimumab is a fully human IgG1 monoclonal antibody (mAb) that binds specifically to TNF-α. The molecule consists of 1330 amino acids and its molecular weight is approximately 148 kDa.[4] It is composed of two H and two L polypeptide chains, with each containing three complementarity-determining regions in the heavy (VH) and light (VL) variable domains. Like the structure of IgG, adalimumab has two antigen-binding Fab domains linked to the Fc domain via a hinge. Six complementarity-determining regions of each H:L chain pair compose the antigen-binding site on the Fab domain of the mAb.[5]
In addition to heavy and light variable regions, adalimumab consists of human IgG1:κ constant regions that are engineered by phage display technology. The phage display facilitates selection of a fully human antibody specific for a specific antigen, in this case tumor necrosis factor (TNF), from a large range of antibodies. If the desired antibody is rare in this range, a two-stage process is applied for a more rapid guided selection. For the generation of adalimumab, the first step is the usage of anti-human TNF murine antibody MAK195 for the isolation of a human antibody that can recognize the same neutralizing epitope as MAK195. This antibody has a low off-rate and high affinity for human TNF. VH and VL MAK195 are paired with human cognate repertoires. For these phage antibody libraries, recombinant human TNF serves as the antigen for the antigen binding selection. A fully human anti-TNF antibody is then generated by combining the selected human VH and VL genes. Early human anti-TNF antibodies were optimized in a second phase that mirrors the natural process for antibody optimization. It is produced in a Chinese hamster ovary host, transfected with a plasmid vector containing the expression cassettes for adalimumab light and heavy chains.[6]
History
The first version of adalimumab was engineered in the 90s and was named D2E7. BASF Pharma started the development of a TNF neutralizing human antibody with the use of phage display technology in 1993 in collaboration with Cambridge Antibody Technology. Phage display repertoires were used to guide the selection of human antibodies to a single epitope of antigen TNF-⍺. This technology was developed in 1991 by Cambridge Antibody Technology. A phage antibody library technology was developed that could be used to discover human antibodies of therapeutic value. To select the antibodies that bind to a desired antigen, enormous repertoires of human antibodies are displayed on the surfaces of millions of bacterial phages, i.e. phage antibody library.[8][9]
The compound that later became adalimumab was identified within two years after the start of the development.[10] In 2002, Abbott received approvement of the Food and Drug Administration (FDA) for sales of Humira for the treatment of rheumatoid arthritis (RA).[1] It became to be the first fully human monoclonal antibody to be approved by the FDA. The patent on Humira belonged to AbbVie, a spin-off from Abbott, and is approved for the treatment of psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, hidradenitis suppurativa, juvenile idiopathic arthritis, uveitis and plaque psoriasis.[4] The patent expired in October 2018 in Europe, which led to the immediate launch of other biosimilars. In the US, AbbVie’s patent on Humira expired in 2016 but the company has managed to prolong its protection until 2023.[11][12]
Mechanism of action
Pathophysiology of Rheumatoid Arthritis
TNF-⍺ is a proinflammatory cytokine and is part of the type II cytokine family. It is a ~26 kDa protein that is produced by activated macrophages, monocytes, and activated T-cells. Synthetization occurs as a transmembrane TNF (tmTNF). The ~15 kDa soluble form of TNF (sTNF) is released after proteolysis by a TNF-⍺ converting enzyme in the extracellular domain. It can bind to its receptors, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). Both receptors are naturally monomeric and occur on cell surfaces and in soluble form.[13][14][15] Both sTNF and tmTNF bind to TNFR1 and TNFR2. The biological functions of TNF-⍺ are expressed by its binding to receptors. One of these biological functions is starting the inflammation process.
The pathophysiology of rheumatoid arthritis (RA) is generated by B- and T-cells, with a prominent role of the pro-inflammatory cytokines TNF-⍺ and IL-1. The permeation of CD4+ T cells into the synovium of the joint plays an important role in the inflammatory process. CD4+ T cells, activated by an antigen, stimulate the production of TNF-⍺, IL-1 and IL-6. This stimulation is the driving force behind ongoing inflammation in RA. It is thought that these CD4+ T cells also stimulate B cells in the production of immunoglobulins such as rheumatoid factor. Rheumatoid factor and IgG can form an immune complex, and this, in turn, contributes to the RA pathogenesis by activation of the complement system.[6]
In patients with RA, elevated TNF-⍺ levels are found in the cartilage-pannus junction and the synovial tissue. It is produced locally in the synovium of joints. TNF-⍺ induces the production and secretion of cartilage-degrading matrix metalloproteinase enzymes (MMPs) from synovial fibroblasts. The MMPs inhibit tissue inhibitors of metalloproteinase. In total, this leads to the breakdown of collagen and joint destruction, due to matrix-degrading activities.[16] Under the influence of pro-inflammatory cytokines, the macrophage colony stimulating factor (M-CSF) and the receptor activator of nuclear factor-kB ligand are activated. As a result, osteoclasts maturate and the osteoprotegerin ratio decreases, which leads to constant osteoclasts differentiation. Matured osteoclasts attached to the matrix, secrete hydrochloric acid and a proteolytic enzyme cathepsin K. These acids and enzymes destroy osteonectin and aggrecan, which result in chronic joint destruction.[17]
Mode of action
Disease-modifying anti-rheumatic drugs (DMARDs) like adalimumab are used to reduce disease progression and to improve function by inhibiting the inflammation process. Adalimumab is a highly specific TNF-⍺ neutralizing antibody. Unlike other mAbs, adalimumab does not bind to other forms of TNF, like lymphotoxin-⍺. With its binding to soluble TNF-⍺, adalimumab inhibits the interaction of TNF-⍺ with TNFR1 and TNFR2 and prevents it from expression its biological function. With its Fab arms, it has the ability to crosslink two trimeric sTNF at the same time, which causes multimeric complexes to form.[5][6]
Adalimumab can also bind tmTNF. In a study that used a system with Jurkat T-cells to study the effect of adalimumab, the drug was found to induce complement-dependent cytotoxicity (CDC). The first component of complement activation (C1) is activated by the CH2 region of the Fc portion, which eventually leads to cell lysis. At the same time, adalimumab induces antibody-dependent cell-mediated cytotoxicity (ADCC). The CH2 and CH3 domains of the Fc domain of IgG1 play a role in the binding of adalimumab to the Fc receptors on a NK cell. This sets the lysis of the target cell in motion by granzyme B and perforin. Moreover, adalimumab is involved in reverse signaling. After the binding of adalimumab to tmTNF, cell apoptosis and cell cycle G0/G1 arrest are induced.[18]
Pharmacokinetics
Adalimumab is administered by the subcutaneous (SC) route. The absorption of adalimumab after SC administration is not fully understood. It is suggested that the absorption occurs like the diffusion of IgG across blood vessels and its relocation through lymphatic vessels. Flow through lymphatic vessels is slow, which causes the adsorption to last several days with a large interindividual variability.[19] Peak serum concentrations of adalimumab are reached after around 5 days post administration. The absolute bioavailability after a single 40-mg dose was 64%. Concentrations of adalimumab in the synovial fluid of RA patients ranged from 31-96% of those in serum.[4]
As adalimumab is a large hydrophilic molecule, it should be confined to lymphatic vessels and blood vessels and report low tissue penetration. However, adalimumab penetrates cells through fluid phase endocytosis or through receptor-mediated endocytosis. Size prevents it from glomerular filtration and adalimumab is thus not eliminated via renal or biliary excretion. Elimination of adalimumab occurs non-specific (linear). The half-life is around twenty days.[3] This long serum half-life can be explained by the binding of neonatal Fc receptors on endothelial cells to the Fc domain of IgG at acidic pH. This protects IgG from catabolic activities and contributes to its long half-life. As adalimumab is much like IgG, it is thought that it goes through the same process as IgG.[7] The mechanisms by which antibodies are cleared from the circulation are not fully understood.[19]
Interpatient variability of the pharmacokinetics can be explained by several factors, including antigenic burden, the presence of anti-drug antibodies (ADAbs) and body size. For most mAbs, the clearance and volume of distribution of mAbs increase with body size. These parameters are also reported to be higher in men than in women. For adalimumab, the clearance rate is around 40% higher in men. As the response to adalimumab is reported to increase with serum concentrations, a dosage adjustment by the influence of body weight and body surface area can be justified.[3][19]
Efficacy
Adalimumab is an overall well-tolerated drug. However, some patients show an inadequate response to the drug. Around 10–30% of patients do not respond to the initial treatment and 23–46% of patients lose response over time.[20] Because adalimumab is an exogenous protein, it can induce an immune response. While the risk of the formation of anti-drug antibodies (ADAb) are very high for murine mAbs, the risk is also present for human antibodies like adalimumab. A decreased response in treatment of adalimumab can be associated with the formation of ADAb.[19] For patients that show an inadequate response to adalimumab and by whom the presence of ADAb is registered, an increase in the dosage or dose frequency of adalimumab can lead to a decrease in detectable ADAb. By increasing the dosing frequency, it might overload the ability of the immune system to produce sufficient ADAb or it may induce immunotolerance to adalimumab.[21] This could potentially increase clinical outcome.
Not only the presence of ADAb is a predictor of a non-response. Some non-responders have no detectable ADAb or have adequate or high drug levels without clinical response. In patients without detectable drug levels, however, no clinical effect of adalimumab was registered.[19][22]
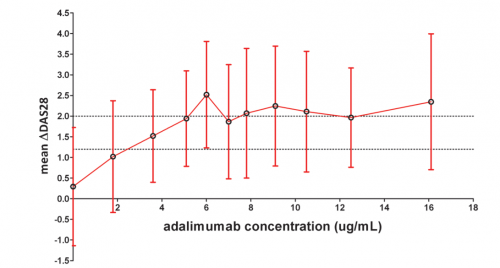
A good clinical response is characterized by a Disease Activity Score in 28 joints (DAS28) improvement of 1.2 and higher. Serum concentrations of around 3 µg/mL are sufficient to reach this threshold. Serum levels up to 8 µg/mL have a positive effect on the DAS28 score, therefore the probability of clinical response to adalimumab increases with the trough serum concentrations. It should be noted that serum levels surpassing 8 µg/mL do not contribute to further clinical improvement. The cut-off value to distinguish between good responders and non and moderate of responders was determined to be 5 µg/mL. Therefore, adalimumab serum trough concentrations in the range of 5-8 µg/mL were found to be predictive of good clinical response. A DAS28 improvement score below 0.6 is classified as a no response.[22]
| DAS28 improvement | ||||
|---|---|---|---|---|
| >1.2 | >0.6 and ≤ 1.2 | ≤0.6 | ||
| Present DAS28 score | ≤3.2 | good response | moderate response | no response |
| >3.2 and ≤5.1 | moderate response | moderate response | no response | |
| >5.1 | moderate response | no response | no response | |
The use of serum trough levels along with an assessment of disease activity, can serve as an early prediction of response to adalimumab in RA. The level reached in serum depends on factors like adsorption rate, distribution rate and clearance. Furthermore, these factors are influenced by physical states like gender, age and disease state.[22]
For mAbs in general, patients with high disease activity have an increased amount of TNF-⍺. Due to target-mediated elimination, this increase leads to a increased target-mediated clearance which in turn leads to lower concentrations of TNF targeting mAbs. Consequently, patients that express a high disease activity have a lower exposure to anti-TNF-⍺ mAbs. RA patients should therefore receive higher mAb doses according to disease activity. To solidify this and to propose rational treatment strategies, more pharmacokinetic-pharmacodynamic studies are needed.[19]
As the half-life of adalimumab is around twenty days, steady state is only achieved after 4 months after initiation of treatment. No loading dose is recommended for RA patients and therefore the time to reach steady-state is delayed. Instituting a higher loading dose to reach steady state faster could potentially decrease the risk of a non-response. This is seen in Crohn’s disease patients, in whom the use of loading doses was shown to increase treatment efficacy and decrease the risk of developing ADAb.[3]
Safety
Biological DMARDs like adalimumab have a unique mechanism of action. Adalimumab blocks the overexpressed cytokine TNF-⍺ and with its binding, inhibits an important signaling protein in the normal immune response. TNF plays a critical role in the formation of post-infectious granuloma and its maintenance, which are important components for the host defenses against microbial pathogens, such as Mycobacterium Tuberculosis, histoplasmosis and other opportunistic infections. Deactivation of TNF can give way to granulomatous infectious diseases, like tuberculosis, histoplasmosis, and other less common conditions.[14][24]
The most common adverse effects of the usage of adalimumab are infections and immunological reactions, like hypersensitivity, injection-site and infusion-related reactions. More serious adverse events include the reactivation of tuberculosis and hepatitis B. Therefore, patients with a history of chronic infections and recurrent infections should avoid biologic therapies. Adalimumab is contra-indicated for patients with sepsis or who are at risk of sepsis, with active tuberculosis or other opportunistic infections.[4] Some studies report an increased risk of infections with higher doses of adalimumab. However, this correlation is not completely certain.[19][25] Therefore, caution has to be taken with prescribing DMARDs and for prescribing the right dosage
Lab protocols
Adalimumab in solution should be refrigerated between 2ºC to 8ºC and protected from light. It should not be frozen.[4] It can be harmful if inhaled and cause respiratory tract irritation. To prevent harmful absorption through the skin and prevent possible skin irritation, protective gloves are recommended. Moreover, swallowing the compound could be harmful.[26]
Samples containing adalimumab have to be analyzed within 72 hours. Otherwise the samples must be frozen at ≤ -18 °C for 12 months. Disposal of samples are to be performed according to your laboratory regulations.[27]
Medical Use and TDM
The drug is supplied as a solution for injection with a pH of 5.2. Current available dosage forms are 40 mg/0.8 mL, 40 mg/0.4 mL, 20 mg/0.4 mL and 10 mg/0.2 mL single-use prefilled syringe. There also exist prefilled pens of 40 mg/0.8 mL and 40 mg/0.4 mL.[4] It is administered once every week or every other week. The target steady state trough concentration is between 5 and 8 µg/mL.
In current practices, when the concentration of adalimumab is at a normal range, but the patient has no decreased disease activity, the patient can be classified as non-responding. A switch to a different medicine could be beneficial. When serum levels of adalimumab are too low, the underlying cause, either non-compliance or ADAb, is investigated. If the concentration is not extremely low, an increase of dosage can be considered.
Measurements of adalimumab levels are not routinely done. Therapeutic drug monitoring (TDM) is the practice of measuring the concentration of a specific drug in the bloodstream with the aim of using this data to optimize the individual dosing schemes of patients. TDM could provide a means to optimize the treatment with adalimumab. The introduction of a biosensor for adalimumab as a means for TDM would allow for better drug monitoring. It gives the possibility to detect non-responders in an early stage of treatment or to optimize dosing strategies. For patients with too high serum levels, dosage reduction to obtain serum levels between 5 and 8 µg/mL could be beneficial for the patient as the expensive drug is used more optimally. As ADAb can be the cause of the lower adalimumab serum levels, measurement of ADAb with a biosensor is also a possibility. However, as adalimumab can interfere with an assay that measures ADAb, measurement of adalimumab itself is to be prefered.[22]
A treatment based on TDM has the potential to ensure a maximal clinical benefit with the lowest dose of the drug.[22] Next to the health benefit, treatment could have an economic benefit, as it can be cost-saving in the long-term, especially in the case of dose reduction.
A possible difficulty in the implementation of personalized dosing schemes are the fixed dosing regimens. Drugs that are administered via SC route are provided in fixed dosing regimens, for example a 40-mg prefilled syringe. In case of intravenous dosing (IV), dosing adjustments for body size and disease activity are possible for anti-TNF-⍺ mAbs.[19]
State of the art of adalimumab assays
At the moment, there are no handheld or table-top point-of-care devices for detecting adalimumab available on the market. In the table below, a selection of the available adalimumab assays is listed.
| Company | Product | Test name | Sample Volume | Reportable range | Dilution | Precision | Incubation time |
|---|---|---|---|---|---|---|---|
| Sanquin[27] | M2910 | MabTrack level adalimumab | 5 μL | 1–30 µg/mL | 1:200 1:1500 1:2000 |
Total CV < 15% Inter-assay: CV < 15.4% |
2 hours 10 min. |
| R-Biopharm AG[28] | G09043 | RIDASCREEN ADM Monitoring | 10 μL | 0.5-12 µg/mL 2.0- 48 µg/mL |
1:100 1:400 |
Intra-assay: CV < 10.1% Inter-assay: CV< 14.2% |
1 hour 40 min. |
| Theradiag[29] | LTA 002 | LISA-TRACKER Adalimumab | 5 μL | 0.3 - 16 µg/mL | 1:201 | Intra-assay: CV < 13.3% Inter-assay: CV< 9.7% |
2 hours |
| R-Biopharm AG[30] | GN3043 | RIDA®QUICK ADM Monitoring | 20 μL | 0.5 - 25 μg/ml | 1:441 | Intra-assay: CV < 16.8% Inter-assay: CV< 16.6% |
15 min. |
| BÜHLMANN[31] | LF-TLAD25 | Quantum Blue® Adalimumab | 10 μL | 1.3 - 35 μg/mL | 1:190 | Intra-assay: CV < 28.6% Inter-assay: CV < 12.6% |
15 min |
To determine the trough levels of adalimumab, the samples must be taken within 24 hours prior to the drug administration.[27]
These assays are sandwich-type assays with enzymatic labelling, expect for the RIDA®QUICK ADM Monitoring and Quantum Blue® Adalimumab, which are based on lateral flow immunoassays. Mabtrack by Sanquin uses polystyrene microtiter wells with immobilized TNF-specific mouse monoclonal antibodies. These bind recombinant TNF. The adalimumab that is present in the sample bind to the bound TNF on the microtiter plate and a adalimumab/TNF/anti-TNF complex is formed. Next, a monoclonal ADAb labeled with horseradish peroxidase is added which binds to the complex. A substrate solution leads to the formation of a colored product, proportional to the amount of adalimumab present in the sample.[27] The RIDASCREEN ADM Monitoring and LISA-TRACKER Adalimumab both have immobilized TNF-⍺ on the surface of the microwell plate. While RIDASCREEN ADM makes use of ADAb conjugated with horseradish peroxidase to form a TNF-⍺/adalimumab/conjugated-ADAb complex, the LISA-TRACKER uses anti-human IgG biotinylated antibodies to form the complex, whereafter horseradish peroxydase labelled with streptavidin is added that binds to the complex.[28][29]
Numbers
Rheumatoid arthritis affects around 1% of the world population per year.[32] Global estimates in 2010 reported a prevalence rate of 0.35% for women and 0.13% for men. The prevalence of RA is higher in more developed countries.[33] A study in the US reported an overall lifetime risk for RA of 3.6% for women and 1.7% for men. This corresponds to around 1 in 28 women and 1 in 59 men that will develop RA in their lifetime.[34]
Globally in 2010, RA represented 0.49% of years lived with disability (YLD) and 0.19% of disability-adjusted-years (DALY). Across 31 nations in the period of 2009–2011, a total of 219,189 patients died, in whom RA was registered as the underlying cause of death. The YLDs for RA were 55/100000 population and the total DALYs were around 4.8 million.[33]
The US reported having approximately $128 billion of direct and $47.0 billion of indirect costs billable to arthritis and related rheumatic conditions. In the UK, this number came down to £560 million a year in health care costs.[33] In 2017, adalimumab (Humira) was at the top of pharmaceutical products by sales worldwide. The drug generated more than 18.4 billion US dollars. Almost twice as much as Rituxan, who took second place with 9.2 billion dollars generated.[35] In 2015, Humira costs around $2669 per month in the US and $1362 in the UK.[36]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Drugbank (2005). Adalimumab. Accessed on 6 November 2018, at https://www.drugbank.ca/drugs/DB00051.
- ↑ U.S. National Library of Medicine (2018). Adalimumab. Accessed on 6 November 2018, at https://chem.nlm.nih.gov/chemidplus/rn/331731-18-1.
- ↑ 3.0 3.1 3.2 3.3 Ternant, D. et al. (2014). Pharmacokinetics and concentration–effect relationship of adalimumab in rheumatoid arthritis. British Journal of Clinical Pharmacology, 79(2), 286–297. doi:10.1111/bcp.12509
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Medsafe (2012). Humira solution for injection: Data Sheet. Accessed on 6 November 2018, at http://www.medsafe.govt.nz/Medicines/SearchResult.asp.
- ↑ 5.0 5.1 Tracey, D., Klareskog, L., Sasso, E.H., Salfeld, J.G., Tak, P.P. (2008). Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacology & Therapeutics, 117(2), 244-279. doi:10.1016/j.pharmthera.2007.10.001
- ↑ 6.0 6.1 6.2 Boehncke, W.H., Radeke, H.H. (2007). Adalimumab. In Salfeld,J., Kupper, H., Biologics in General Medicine., 14-31, Springer-Verlag Berlin Heidelberg. doi:10.1007/978-3-540-29018-6
- ↑ 7.0 7.1 Mitoma, H., Horiuchi, T., Tsukamoto, H., Ueda, N. (2018). Molecular mechanisms of action of anti-TNF-α agents – Comparison among therapeutic TNF-α antagonists. Cytokine, 101, 56-63. doi:10.1016/j.cyto.2016.08.014
- ↑ Jespers, L.S., Roberts, A., Mahler, S.M., Winter, G., Hoogenboom, H.R. (1994). Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Bio/Technology, 12(9), 899–903. doi:10.1038/nbt0994-899
- ↑ Den Broeder, A. et al. (2002). A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. The Journal of Rheumatology, 29(11), 2288-2298
- ↑ McCafferty, J. (2010). The long and winding road to antibody therapeutics,mAbs, 2(5), 459-460. doi:10.4161%2Fmabs.2.5.13088
- ↑ Loftus, P. (2017). AbbVie, Amgen Reach Settlement in Humira Patent Dispute, The Wall Street Journal. Retrieved from https://www.wsj.com/articles/abbvie-amgen-reach-settlement-in-humira-patent-dispute-1506635070.
- ↑ Derbyshire, M. (2015). Patent expiry dates for best-selling biologicals, Generics and Biosimilars Initiative Journal, 4(4), 178-179. doi:10.5639/gabij.2015.0404.040.
- ↑ Deora, A. et al. (2017). Transmembrane TNF-dependent uptake of anti-TNF antibodies. MAbs, 9(4), 680-695. doi:10.1080/19420862.2017.1304869
- ↑ 14.0 14.1 Haraoui, B., Bykerk, V. (2007). Etanercept in the treatment of rheumatoid arthritis. Therapeutics and clinical risk management, 3(1), 99–105. doi:10.2147/tcrm.2007.3.1.99
- ↑ Wang, J., Al-Lamki, R.S. (2013). Tumor Necrosis Factor Receptor 2: Its Contribution to Acute Cellular Rejection and Clear Cell Renal Carcinoma. BioMed Research International, 2013(1), 1-11. doi:10.1155/2013/821310
- ↑ Alldred, A. (2001). Etanercept in rheumatoid arthritis. Expert Opinion on Pharmacotherapy, 2(7), 1137-1148. doi:10.1517/14656566.2.7.1137
- ↑ Fazal, S.A. et al. (2018). A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. Endocrine, Metabolic & Immune disorders - Drug Targets, 18(2), 98-109. doi:10.2174/1871530317666171114122417
- ↑ Horiuchi, T., Mitoma, H., Harashima, S., Tsukamoto, H., Shimoda, T. (2010). Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology, 49(7), 1215–1228. doi:10.1093/rheumatology/keq031
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Ternant, D., Bejan-Angoulvant, T., Passot, C., Mulleman, D., Paintaud, G. (2015). Clinical Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies Approved to Treat Rheumatoid Arthritis. Clinical Pharmacokinetics, 54(11), 1107-1123. doi:10.1007/s40262-015-0296-9
- ↑ Roda, G., Jharap, B., Neeraj, N., Colombel, J.F. (2016). Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clinical and translational gastroenterology, 7(1), e135. doi:10.1038/ctg.2015.63
- ↑ Bartelds, G.M. et al. (2007). Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Annals of the Rheumatic Diseases, 66(1), 921-926. doi:10.1136/ard.2006.065615
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Pouw, M.F. et al. (2015). Key findings towards optimising adalimumab treatment: the concentration–effect curve. Annals of the rheumatic diseases, 74(3), 513–518. doi:10.1136/annrheumdis-2013-204172
- ↑ Fransen, J., Van Riel, P.L.C.M. (2005) The Disease Activity Score and the EULAR response criteria, Clinical and Experimental Rheumatology, 23(39), S93-S99.
- ↑ Wallis, R.S., Broder, M.S., Wong, J.Y., Hanson, M.E., Beenhouwer, D.O. (2004). Granulomatous Infectious Diseases Associated with Tumor Necrosis Factor Antagonists. Clinical Infectious Diseases, 38(9), 1261–1265. doi:10.1086/383317
- ↑ Bongartz, T., Sutton, A.J., Sweeting, M.J,, Buchan, I., Matteson, E.L., Montori, V. (2016). Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies Systematic Review and Meta-analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA, 295(19), 2275-2285. doi:10.1001/jama.295.19.2275
- ↑ BioVision (2018). Anti-TNF-a (Adalimumab): Datasheet. Accessed on 9 November 2018, at https://www.biovision.com/anti-tnf-adalimumab-humanized-antibody.html.
- ↑ 27.0 27.1 27.2 27.3 MabTrack level adalimumab (2018). Leaflet MabTrack level adalimumab. Sanquin.
- ↑ 28.0 28.1 RIDASCREEN ADM Monitoring (2016). Leaflet RIDASCREEN ADM Monitoring. R-Biopharm AG.
- ↑ 29.0 29.1 LTA002 LISA-TRACKER Adalimumab (2017). Leaflet LTA002 LISA-TRACKER Adalimumab. Theradiag.
- ↑ GN3043 RIDA®QUICK ADM Monitoring (2018). Leaflet GN3043 RIDA®QUICK ADM Monitoring. R-Biopharm AG.
- ↑ LF-TLAD25 Quantum Blue® Adalimumab (2018). Leaflet LF-TLAD25 Quantum Blue® Adalimumab. BÜHLMANN.
- ↑ Marita Cross, M. et al. (2014). The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Annals of Rheumatic diseases, 73, 1316-1322. doi:10.1136/annrheumdis-2013-204627
- ↑ 33.0 33.1 33.2 Fazal, S.A. et al. (2018). A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. Endocrine, Metabolic & Immune disorders - Drug Targets, 18(2), 98-109. doi:10.2174/1871530317666171114122417
- ↑ Crowson, C.S. et al. (2011). The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis and Rheumatism, 63(3), 633-639. doi:10.1002/art.30155
- ↑ Statista (2018). Top 15 pharmaceutical products by sales worldwide in 2017. Accessed on 7 November 2018, at https://www.statista.com/statistics/258022/top-10-pharmaceutical-products-by-global-sales-2011/.
- ↑ Statista (2018). Average prices of Humira in selected countries in 2015. Accessed on 7 November 2018, at https://www.statista.com/statistics/312014/average-price-of-humira-by-country/.