Difference between revisions of "Acute Inflammation with a Focus on Sepsis"
Techsensus (talk | contribs) (→History of Sepsis) |
Techsensus (talk | contribs) (→Mechanism of Acute Inflammation) |
||
| Line 20: | Line 20: | ||
== Mechanism of Acute Inflammation == | == Mechanism of Acute Inflammation == | ||
| − | When the human body is subjected to harmful stimuli, an inflammatory response is activated to remove these stimuli and, if necessary, initiate a healing process. Cellular and molecular events take place to minimize injury and infection. Common characteristics of inflammation on tissue level are redness, heat, pain and loss of tissue function, which all result from local immune, vascular and inflammatory cell responses to infection or injury.<ref name="Arti11">Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9(6), 7204–7218, 2018, Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L., https://doi.org/10.18632/oncotarget.23208 </ref>The mentioned events are a consequence of specific complex molecular pathways involving different types of receptors, transcription factors, leukocytes and eventually cytokines that induce inflammatory responses. Even though different stimuli may evoke different inflammation pathways in the human body, in general, a common mechanism is applied which can be summarized in four steps as stated in research done by Chen et al., 2018 <ref name="Arti11">Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9(6), 7204–7218, 2018, Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L., https://doi.org/10.18632/oncotarget.23208 </ref>: | + | When the human body is subjected to harmful stimuli, an inflammatory response is activated to remove these stimuli and, if necessary, initiate a healing process. Cellular and molecular events take place to minimize injury and infection. Common characteristics of inflammation on tissue level are redness, heat, pain and loss of tissue function, which all result from local immune, vascular and inflammatory cell responses to infection or injury.<ref name="Arti11">Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9(6), 7204–7218, 2018, Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L., https://doi.org/10.18632/oncotarget.23208 </ref> The mentioned events are a consequence of specific complex molecular pathways involving different types of receptors, transcription factors, leukocytes and eventually cytokines that induce inflammatory responses. Even though different stimuli may evoke different inflammation pathways in the human body, in general, a common mechanism is applied which can be summarized in four steps as stated in research done by Chen et al., 2018 <ref name="Arti11">Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9(6), 7204–7218, 2018, Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L., https://doi.org/10.18632/oncotarget.23208 </ref>: |
1. Cell surface pattern receptors recognize harmful stimuli | 1. Cell surface pattern receptors recognize harmful stimuli | ||
| Line 27: | Line 27: | ||
4. Inflammatory cells are recruited | 4. Inflammatory cells are recruited | ||
| − | Pathogen-associated molecular patterns (PAMPs) trigger inflammatory responses through activation of specific pattern recognition receptors. As a result, the production of proinflammatory cytokines is induced. Proinflammatory cytokines are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions.<ref name="Arti12">Cytokines, inflammation, and pain. International Anesthesiology Clinics, 45(2), 27–37, 2007, Zhang, J.-M., & An, J., https://doi.org/10.1097/AIA.0b013e318034194e </ref>Interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are cytokines that mediate receptor activation in order to trigger crucial intracellular signaling pathways that may start the healing process. | + | Pathogen-associated molecular patterns (PAMPs) trigger inflammatory responses through activation of specific pattern recognition receptors. As a result, the production of proinflammatory cytokines is induced. Proinflammatory cytokines are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions.<ref name="Arti12">Cytokines, inflammation, and pain. International Anesthesiology Clinics, 45(2), 27–37, 2007, Zhang, J.-M., & An, J., https://doi.org/10.1097/AIA.0b013e318034194e </ref> Interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are cytokines that mediate receptor activation in order to trigger crucial intracellular signaling pathways that may start the healing process. |
| − | However, in the case of acute inflammation, the response to an infection is dysregulated and often disproportional to the severity of the infection. The response gets overheated, overactivated, and can damage the body from within. Potential consequences of this overly strong reaction include infections, organ dysfunction (severe sepsis), or septic shock which is a state of circulatory failure where circulatory, cellular and metabolic abnormalities are associated with an increased risk of death. These reactions are often caused by coagulation (i.e. formation of blood clots) dysregulation. The hypercoagulability of sepsis is thought to be driven by the release of tissue factor from disrupted endothelial cells.<ref name="Arti13">Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Medicine, 7, 2050312119835043–2050312119835043, 2019, Gyawali, B., Ramakrishna, K., & Dhamoon, A. S., https://doi.org/10.1177/2050312119835043 </ref>When the human body suffers from severe sepsis, activated monocytes and endothelial cells, along with circulating microvesicles, become sources of tissue factor<ref name="Arti14">Role of extracellular vesicles in the development of sepsis-induced coagulopathy. Journal of Intensive Care, 6, 68, 2018, Iba, T., & Ogura, H., https://doi.org/10.1186/s40560-018-0340-6 </ref>. | + | However, in the case of acute inflammation, the response to an infection is dysregulated and often disproportional to the severity of the infection. The response gets overheated, overactivated, and can damage the body from within. Potential consequences of this overly strong reaction include infections, organ dysfunction (severe sepsis), or septic shock which is a state of circulatory failure where circulatory, cellular and metabolic abnormalities are associated with an increased risk of death. These reactions are often caused by coagulation (i.e. formation of blood clots) dysregulation. The hypercoagulability of sepsis is thought to be driven by the release of tissue factor from disrupted endothelial cells.<ref name="Arti13">Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Medicine, 7, 2050312119835043–2050312119835043, 2019, Gyawali, B., Ramakrishna, K., & Dhamoon, A. S., https://doi.org/10.1177/2050312119835043 </ref> When the human body suffers from severe sepsis, activated monocytes and endothelial cells, along with circulating microvesicles, become sources of tissue factor<ref name="Arti14">Role of extracellular vesicles in the development of sepsis-induced coagulopathy. Journal of Intensive Care, 6, 68, 2018, Iba, T., & Ogura, H., https://doi.org/10.1186/s40560-018-0340-6 </ref>. |
-- | -- | ||
| − | This factor then causes the systemic activation of the coagulation cascade resulting in the production of thrombin, activation of platelets, and formation of platelet–fibrin clots. These structures can result in local perfusion defects leading to tissue hypoxia and organ dysfunction | + | This factor then causes the systemic activation of the coagulation cascade resulting in the production of thrombin, activation of platelets, and formation of platelet–fibrin clots. These structures can result in local perfusion defects leading to tissue hypoxia and organ dysfunction <ref name="Arti13">Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Medicine, 7, 2050312119835043–2050312119835043, 2019, Gyawali, B., Ramakrishna, K., & Dhamoon, A. S., https://doi.org/10.1177/2050312119835043 </ref>. Moreover, research has shown that dysregulated apoptotic immune cell-death plays a crucial part in immune dysfunction and mortality of sepsis. Apoptosis is a “programmed cell death” to limit damage of surrounding tissue during the immune response<ref name="Arti15">Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4), 495–516, 2007, Elmore, S., https://doi.org/10.1080/01926230701320337 </ref>. It is a vital component of many processes in the human body such as cell turnover, proper development and functioning of the immune system ref name="Arti15">Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4), 495–516, 2007, Elmore, S., https://doi.org/10.1080/01926230701320337 </ref>. Most cells that undergo enhanced apoptosis in sepsis are of lymphoid origin, hence less immune cells are left to fight off the infection itself <ref name="Arti16">Host–pathogen interactions in sepsis. The Lancet Infectious Diseases, 8(1), 32–43, 2008, van der Poll, T., & Opal, S. M., https://doi.org/https://doi.org/10.1016/S1473-3099(07)70265-7 </ref>. Since no effective treatment for sepsis exists yet, early diagnosis and recognition is crucial.<ref name="Arti17">Sepsis and septic shock: current approaches to management. Internal Medicine Journal, 49(2), 160–170., 2019, Thompson, K., Venkatesh, B., & Finfer, S., https://doi.org/10.1111/imj.14199 </ref> |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | This is where IL-6 plays an important part. As mentioned, IL-6 is a cytokine that functions as a crucial mediator during the acute phase of response to inflammation in sepsis.<ref name="Arti18">Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infectious Diseases, 19(1), 968, 2019, Song, J., Park, D. W., Moon, S., Cho, H.-J., Park, J. H., Seok, H., & Choi, W. S., https://doi.org/10.1186/s12879-019-4618-7 </ref> Research on the clinical value of IL-6 in patients with sepsis and septic shock describes that IL-6 is considered controversial regarding its diagnostic and prognostic values, where meta-analysis of diagnostic value of IL-6 has shown that IL-6 only offers moderate success in differentiating sepsis from non-infectious systemic inflammatory response syndrome (SIRS).<ref name="Arti19">Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine, 88, 126–135, 2016, Ma, L., Zhang, H., Yin, Y.-L., Guo, W.-Z., Ma, Y.-Q., Wang, Y.-B., Shu, C., & Dong, L.-Q., https://doi.org/10.1016/j.cyto.2016.08.033 </ref> Hence it is recommended that IL-6 is used as a biomarker to confirm infection rather than differentiate between sepsis and SIRS<ref name="Arti19">Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine, 88, 126–135, 2016, Ma, L., Zhang, H., Yin, Y.-L., Guo, W.-Z., Ma, Y.-Q., Wang, Y.-B., Shu, C., & Dong, L.-Q., https://doi.org/10.1016/j.cyto.2016.08.033 </ref>. | ||
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 19:01, 18 November 2021
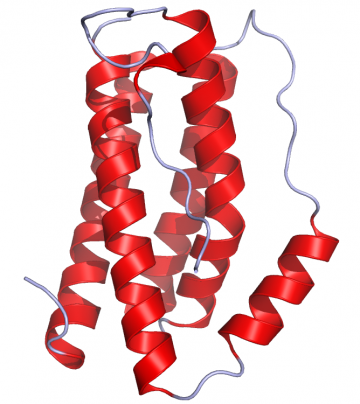
General information
The theme of SensUs 2022 is acute inflammation in intensive care with a focus on sepsis. According to the World Health Organization, sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It causes 11 million deaths each year and affects an estimated 49 million people worldwide.[2] According to experts, the diagnosis of sepsis is often set too late as the symptoms arise when organ injury has already occurred, hence the millions of deaths. Sepsis is one of several acute inflammatory responses; others can be caused by injurious agents such as allergens, toxins, burns, and frostbite.[3] Clinically, acute inflammation is characterized by five cardinal signs: redness, increased heat, swelling, pain, and functio laesa (i.e. loss of function). It may be regarded as the first line of defense against injury, releasing signaling molecules such as cytokines and chemokines to assist in healing the body and returning to homeostasis. [4]
Interleukin (IL)-6 is a pleiotropic cytokine with a wide range of biological activities. It induces pro- and anti-inflammatory reactions and is rapidly induced in the course of acute inflammatory reactions. IL-6 is produced by lymphoid and nonlymphoid cells and helps regulate immune reactivity, the acute phase response, inflammation, oncogenesis, and hematopoiesis. Studies have shown that IL-6 appears to be both a marker and mediator of sepsis and persists in the plasma much longer than other proinflammatory cytokines.[5] Therefore IL-6 serves as a valuable biomarker for the early detection of sepsis.
History of Sepsis
The oldest report that associates sepsis with wounds goes all the way back to a discovery by Edwin Smith. In 1826 he found a papyrus in Luxor, Egypt, which was written around 1600 BC. This papyrus seemed to be a copy of an even older manuscript written around 3000 BC. In this manuscript, 48 cases of traumatic lesions between wounds, fractures, and dislocations are mentioned. Clear references to fever as a secondary phenomenon in the wound – with emphasis on the fever as a part of the monitoring of the patients’ evolution - are found in five out of the forty-eight references. Thus, without being familiar with the concept of infection or inflammation, these Egyptian physicians were able to identify some clear signs of what we know nowadays as local suppuration and systemic infection.[6][7][8]
The first reported use of the word sepsis (σηψις) is in a poem of Homer in the Iliad, where it is a derivative of the word sepo (σηπω), which translates to “I rot”. Yet, the first use of sepsis in a medical context can be found in the Hippocratic corpus, written around 400 BC. The use of this word was related to the phenomenon discovered by the Egyptians. Hippocrates described sepsis as a dangerous odiferous biological decay that could occur in the body. Furthermore, it was believed that this decay took place in the colon and from there “dangerous principles” were released, which could cause “auto-intoxication”. Hippocrates was the first one to try and find antisepsis properties and potential medicinal compounds.[6][9][8]
In the nineteenth century, large growth in the knowledge on the origin and transmission of infectious diseases occurred. One of the physicians who contributed significantly to this development was Ignaz Semmelweiss (1818-1865). He was a physician in Vienna, Austria. In 1841, while working on a maternity ward in a hospital he noticed that there was a high rate of death from childbed fever. Nowadays this is also known as puerperal sepsis. He made the observation that women whose deliveries were assisted by midwives had a significantly lower percentage of infection (2%) than deliveries assisted by medical students (16%). Back then, the medical students practiced both autopsies and childbirth deliveries on the same day without washing their hands. When one of Semmelweis’ colleagues died of an infection, Semmelweis made the connection between the medical students, the deliveries, the autopsies, and puerperal sepsis. Semmelweis’ comment on this situation was “The fingers and hands of students and doctors, soiled by recent dissections, carry those death-dealing cadaver’s poisons into the genital organs of women in childbirth”. When a handwashing policy was implemented, the rates of puerperal sepsis dropped to under 3%.[9][7][8]
In 1964 Dr. Edward Frank from Boston published a management strategy for septic shock. This strategy consisted of continuous monitoring of systemic arterial pressure, central venous pressure, cardiac output, urinary output, blood volume, blood chemistries, gases, pH and electrolytes. Some of these are still used nowadays, such as blood monitoring and urinary output. Aided by the discovery of antibiotics by Alexander Fleming, it was also recommended to find the cause of the infection.[10]
Mechanism of Acute Inflammation
When the human body is subjected to harmful stimuli, an inflammatory response is activated to remove these stimuli and, if necessary, initiate a healing process. Cellular and molecular events take place to minimize injury and infection. Common characteristics of inflammation on tissue level are redness, heat, pain and loss of tissue function, which all result from local immune, vascular and inflammatory cell responses to infection or injury.[11] The mentioned events are a consequence of specific complex molecular pathways involving different types of receptors, transcription factors, leukocytes and eventually cytokines that induce inflammatory responses. Even though different stimuli may evoke different inflammation pathways in the human body, in general, a common mechanism is applied which can be summarized in four steps as stated in research done by Chen et al., 2018 [11]:
1. Cell surface pattern receptors recognize harmful stimuli 2. Inflammatory pathways are activated 3. Inflammatory markers are released 4. Inflammatory cells are recruited
Pathogen-associated molecular patterns (PAMPs) trigger inflammatory responses through activation of specific pattern recognition receptors. As a result, the production of proinflammatory cytokines is induced. Proinflammatory cytokines are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions.[12] Interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are cytokines that mediate receptor activation in order to trigger crucial intracellular signaling pathways that may start the healing process.
However, in the case of acute inflammation, the response to an infection is dysregulated and often disproportional to the severity of the infection. The response gets overheated, overactivated, and can damage the body from within. Potential consequences of this overly strong reaction include infections, organ dysfunction (severe sepsis), or septic shock which is a state of circulatory failure where circulatory, cellular and metabolic abnormalities are associated with an increased risk of death. These reactions are often caused by coagulation (i.e. formation of blood clots) dysregulation. The hypercoagulability of sepsis is thought to be driven by the release of tissue factor from disrupted endothelial cells.[13] When the human body suffers from severe sepsis, activated monocytes and endothelial cells, along with circulating microvesicles, become sources of tissue factor[14].
--
This factor then causes the systemic activation of the coagulation cascade resulting in the production of thrombin, activation of platelets, and formation of platelet–fibrin clots. These structures can result in local perfusion defects leading to tissue hypoxia and organ dysfunction [13]. Moreover, research has shown that dysregulated apoptotic immune cell-death plays a crucial part in immune dysfunction and mortality of sepsis. Apoptosis is a “programmed cell death” to limit damage of surrounding tissue during the immune response[15]. It is a vital component of many processes in the human body such as cell turnover, proper development and functioning of the immune system ref name="Arti15">Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4), 495–516, 2007, Elmore, S., https://doi.org/10.1080/01926230701320337 </ref>. Most cells that undergo enhanced apoptosis in sepsis are of lymphoid origin, hence less immune cells are left to fight off the infection itself [16]. Since no effective treatment for sepsis exists yet, early diagnosis and recognition is crucial.[17]
This is where IL-6 plays an important part. As mentioned, IL-6 is a cytokine that functions as a crucial mediator during the acute phase of response to inflammation in sepsis.[18] Research on the clinical value of IL-6 in patients with sepsis and septic shock describes that IL-6 is considered controversial regarding its diagnostic and prognostic values, where meta-analysis of diagnostic value of IL-6 has shown that IL-6 only offers moderate success in differentiating sepsis from non-infectious systemic inflammatory response syndrome (SIRS).[19] Hence it is recommended that IL-6 is used as a biomarker to confirm infection rather than differentiate between sepsis and SIRS[19].
References
- ↑ Crystal structure of IL-6 as published in the Protein Data Bank rendered in Pymol (PDB: 1ALU), 2006, Ramin Herati
- ↑ WHO - Health topics - Sepsis, WHO, 2021, https://www.who.int/health-topics/sepsis#tab=tab_1
- ↑ Acute Inflammatory Response, StatPearls Publishing LLC, 2020, Hannoodee, Sally Nasuruddin, D. N.
- ↑ Concise Pathology (3rd ed.), Appleton & Lange, 1997, Chandrasoma, P., & Taylor, C. R.
- ↑ Interleukin-6. Critical Care Medicine, 33(12 Suppl), S463-5, 2005, Song, M., & Kellum, J. A. https://doi.org/10.1097/01.ccm.0000186784.62662.a1
- ↑ 6.0 6.1 The History of Sepsis from Ancient Egypt to the XIX Century, (M. C. F. P. E.-L. Azevedo (Ed.); p. Ch. 1). IntechOpen, 2012, Botero, J. S. H., https://doi.org/10.5772/51484
- ↑ 7.0 7.1 The last 100 years of sepsis. American Journal of Respiratory and Critical Care Medicine, 173(3), 256–263, 2006, Vincent, J.-L., & Abraham, E., https://doi.org/10.1164/rccm.200510-1604OE
- ↑ 8.0 8.1 8.2 Sepsis History, https://www.news-medical.net/health/Sepsis-History.aspx, 2018, Ryding, S.
- ↑ 9.0 9.1 Sepsis and septic shock: a history. Critical Care Clinics, 25(1), 83–101, viii, 2009, Funk, D. J., Parrillo, J. E., & Kumar, A., https://doi.org/10.1016/j.ccc.2008.12.003
- ↑ The History of Sepsis Management Over the Last 30 Years. Elsevier, 15(2), 116–117, 2014, Zehava L., N., https://daneshyari.com/article/preview/3235901.pdf
- ↑ 11.0 11.1 Inflammatory responses and inflammation-associated diseases in organs, Oncotarget, 9(6), 7204–7218, 2018, Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L., https://doi.org/10.18632/oncotarget.23208
- ↑ Cytokines, inflammation, and pain. International Anesthesiology Clinics, 45(2), 27–37, 2007, Zhang, J.-M., & An, J., https://doi.org/10.1097/AIA.0b013e318034194e
- ↑ 13.0 13.1 Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Medicine, 7, 2050312119835043–2050312119835043, 2019, Gyawali, B., Ramakrishna, K., & Dhamoon, A. S., https://doi.org/10.1177/2050312119835043
- ↑ Role of extracellular vesicles in the development of sepsis-induced coagulopathy. Journal of Intensive Care, 6, 68, 2018, Iba, T., & Ogura, H., https://doi.org/10.1186/s40560-018-0340-6
- ↑ Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4), 495–516, 2007, Elmore, S., https://doi.org/10.1080/01926230701320337
- ↑ Host–pathogen interactions in sepsis. The Lancet Infectious Diseases, 8(1), 32–43, 2008, van der Poll, T., & Opal, S. M., https://doi.org/https://doi.org/10.1016/S1473-3099(07)70265-7
- ↑ Sepsis and septic shock: current approaches to management. Internal Medicine Journal, 49(2), 160–170., 2019, Thompson, K., Venkatesh, B., & Finfer, S., https://doi.org/10.1111/imj.14199
- ↑ Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infectious Diseases, 19(1), 968, 2019, Song, J., Park, D. W., Moon, S., Cho, H.-J., Park, J. H., Seok, H., & Choi, W. S., https://doi.org/10.1186/s12879-019-4618-7
- ↑ 19.0 19.1 Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine, 88, 126–135, 2016, Ma, L., Zhang, H., Yin, Y.-L., Guo, W.-Z., Ma, Y.-Q., Wang, Y.-B., Shu, C., & Dong, L.-Q., https://doi.org/10.1016/j.cyto.2016.08.033