Contents
General information
The theme of SensUs 2023 is Traumatic Brain Injury (TBI). It is often stated in the literature that TBI is a silent epidemic with an estimated 64–74 million new cases presenting each year.[2] The impairments suffered by many TBI patients, such as memory loss, cognitive dysfunction, or behavioural disturbance, are often not visible. The economic and social impact is considerable, with an estimate of direct medical expenditures and indirect costs (e.g., loss of productivity) attributed to TBI exceeding $60 billion in 2000 in the USA. TBI is defined as an alteration in brain function, or other evidence of brain pathology, caused by an external force.[3] The severity of injury in TBI is classified as mild, moderate, or severe with mild TBIs being the most common.[4] Alteration in brain function can be manifest by loss or decreased level of consciousness, alteration in mental state, incomplete memory for the event, or neurological deficits. Examples of external forces include the head striking or being struck by an object, rapid acceleration or deceleration of the brain, penetration of the brain by a foreign object, and exposure to forces associated with blasts.
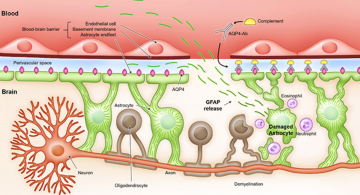
GFAP is a protein found in the glial cells of neural tissue. Glial cells are non-neuronal cells that provide physical and metabolic support to neurons. Blood biomarker levels of GFAP reflect acute injury to brain tissue since the biomarker levels increase strongly and fast within hours after the trauma occurred. Therefore, it has potential use in the emergency unit and intensive care unit, directly after the TBI. Furthermore, the biomarker persists for an extended period with a half-life of 48 hours, making it a favourable biomarker to use in both the acute and subacute phases of injury. The concentration of GFAP peaks at about 20 hours after the injury. Reference serum levels of GFAP range from 0.02 — 0.35 𝜇g/L and the cut-off value of a negative CT scan for GFAP is 0.35 𝜇g/L.[5]
History of TBI
Pre-historic period
TBIs play an important role in the evolution of humans. Studies of skeletons from the mesolithic period (c. 7000 BC) suggest an inverse relationship between the risk of skull fracture and the progress of civilization. The palaeolithic period is a period in prehistory that occurred over a million years ago. In those times, man's predecessor was a semi-erect hominid now named Australopithecus africanus; a damaged skull from this species found in South Africa reveals the first evidence of brain injury.[6]
One of the earliest methods used for treating TBI is called Trepanation. Trepanning, also known as trepanation, is a surgical intervention in which a hole is drilled or scraped into the human skull. The earliest evidence of trepanation performed by the man himself appears in the Neolithic period. The primary theories for the practice of trepanation in ancient times include spiritual purposes and treatment for epilepsy, headache, head wound, and mental disorders.
Ancient History
The first written evidence of TBI is documented in an ancient Egyptian text known as the Edwin Smith Papyrus which was written around 1650-1550 BC and believed to be a copy of an even older manuscript written around 3000 BC. It describes various head injuries and symptoms and classifies them based on their presentation and tractability.[7] This text is so old that it goes as far as mentioning magic as a last resort in terminal cases. To get an idea of what treatment for a brain injury was like then, bandaging the head wound with meat and applying a honey and oil type of dressing until healed were common methods.[8] There are several biblical references to head injuries which are reported to have occurred in the 12-10th century BC. These references to head injuries include: Sisera’s death at the hands of Jael, skull fractures on Abimelech, and the most famous death of Goliath by David.[9]
Hippocrates of Kos, also known as Hippocrates II, was a Greek physician of the classical period who is considered one of the most outstanding figures in the history of medicine and is referred to as the "Father of Medicine”. The first systematic approach to head injuries was by Hippocrates. The Hippocratic Corpus consisted of 76 Treatises, one of which was "On Injuries of the Head".[10]
Medieval History
In the Middle Ages, physicians further described head injury symptoms and the term concussion became more widespread. Berengario da Carpi, an Italian physician, published one of the first books on head injuries. He categorized head injuries into lacerations, contusions and perforations, each of which could be associated with a fracture. A prime example from the 16th century is also one of history’s most well-known figures. A recent study argues that King Henry VIII of England’s erratic behavior was a result of possible repeated traumatic brain injuries. Researchers have made a compelling case citing notes that describe changes including memory loss, irritability, impulsive nature, and insomnia. All known today as common symptoms of a traumatic brain injury.[11]
Modern Era
It was first suggested in the 18th century that symptoms arising from a head injury are not due to a fractured skull, but to injury of the brain. Percival Pott (1713-1788) was one of the first to emphasize that it was the neurological status and not the skull fracture that determined whether surgical intervention was indicated. One of the most notable instances of brain injury took place in the 1800s. Phineas Gage survived an accident where an iron rod penetrated his head and destroyed a good portion of his left frontal lobe. Prior to his accident, Gage was described as “even-tempered”, but his demeanor shifted significantly afterwards. Due to the definitive nature of his injury and the personality changes that followed, many cite this as the first case illustrating mood and personality shifts directly resulting from a brain injury. The 20th century saw the advancement of technologies that improved treatment and diagnosis such as the development of imaging tools including CT and MRI, and, in the 21st century, diffusion tensor imaging (DTI). The introduction of intracranial pressure monitoring in the 1950s has been credited with beginning the "modern era" of head injury.[12] In the 1970s, awareness of TBI as a public health problem grew, and a great deal of progress has been made since then in brain trauma research, such as the discovery of primary and secondary brain injury. Prevention of TBI has also become immensely important with several efforts made in that direction such as: introduction of helmets in the army and motorcyclists, airbags in motor vehicles etc. The 1990s saw the development and dissemination of standardized guidelines for the treatment of TBI, with protocols for a range of issues such as drugs and management of intracranial pressure.
Mechanism of Traumatic Brain Injury
The mechanical force applied to the head displaces the brain. Depending on the direction and magnitude of the force, certain neurological functions can be disrupted. The damage to the brain within the skull can present as strain, tissue distortion and shearing of axons. The processes for damage control are activated immediately, while the damage continues to evolve. The damage occurs as a result of the cellular and molecular processes taking place in response to mechanical forces. First, the mechanical force disrupts the neuronal membrane, which rapidly increases the extracellular potassium concentration. This activates a positive feedback loop where more potassium ions are released, which leads to a cascade of responses that eventually lead to a period of hyperglycolysis in the brain followed by higher permeability of the Blood-Brain-Barrier (BBB) and the induction of cytotoxic edema in the brain.[13] The latter has a detrimental effect on all cells in the brain tissue.
Very important cell types in the brain, aside from neurons, are the neuroglia. These cell types are broadly recognized as having a supportive role. There are 6 types of neuroglia, each with their own function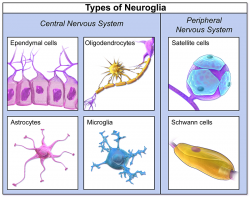
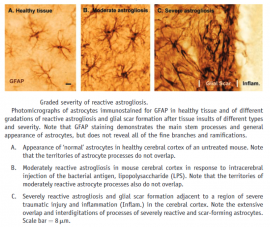
Medical application and relevance
The global incidence of TBI is estimated to be 27 to 69 million a year [20] [21]. Across all severities of TBI, mortality is quite low at 3% [22] this was determined in the United States of America, a country where the healthcare system is quite developed. While mortality is low, the long term effects of TBI can be detrimental to a person’s quality of life. Some of the acute symptoms lessen or resolve over time, such as dizziness or nausea. Other consequences do not become apparent until a long period of time has passed, for instance psychiatric conditions.[23]
Currently, the Glasgow coma scale (GCS) is the only standardized way to assess patients with a suspected TBI. The GCS provides a practical method for assessing impairment of conscious level in response to defined stimuli.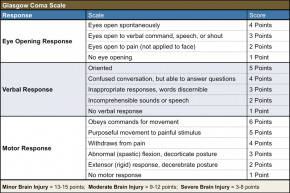
While the GCS score describes the current condition of the patient, its usage has many shortcomings, one of which is the fact that physicians seem to struggle with remembering the exact levels of the scale.[25] Although there is a dose-response relationship with regard to the severity of the TBI and the severity of the outcome, the GCS does not effectively and uniformly predict mortality rate. [26] [27]
Other ways for diagnosing TBI can include various imaging tests like a CT scan or an MRI scan. However, these imaging techniques are often not sensitive/specific enough for milder TBI. According to experts, only 5-10% of mild TBI patients have abnormal CT/MRI scans. The problem with these scans is that they can only detect damage on a macroscopic level, whereas mild TBI manifests primarily at a microscopic level. Therefore, doctors can mistakenly believe that patients with a standard CT or MRI scan have not suffered a TBI.[28] All in all, improvements are needed to objectively and effectively determine whether or not a patient is suffering from a TBI.
State of the Art
Currently, the concentration of GFAP is typically measured in blood plasma (liquid component of blood in which blood cells are absent) or serum (plasma from which the clotting proteins have been removed). Classic methods for the detection of GFAP include ELISA (Enzyme-Linked Immunosorbent Assay).
References
- ↑ Glial Fibrillary Acidic Protein in Blood as a Disease Biomarker of Neuromyelitis Optica Spectrum Disorders. Front. Neurol., 17 March 2022, https://www.frontiersin.org/articles/10.3389/fneur.2022.865730/full#F1
- ↑ Rattani, A., Gupta, S., Baticulon, R. E., Hung, Y. C., Punchak, M., Agrawal, A., Adeleye, A. O., Shrime, M. G., Rubiano, A. M., Rosenfeld, J. V., & Park, K. B. (2019). Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery, 130(4), 1080–1097. https://doi.org/10.3171/2017.10.jns17352
- ↑ Menon, D. K., Schwab, K., Wright, D. W., & Maas, A. I. (2010). Position Statement: Definition of Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation, 91(11), 1637–1640. https://doi.org/10.1016/j.apmr.2010.05.017
- ↑ Iaccarino, C., Gerosa, A., & Viaroli, E. (2021). Epidemiology of Traumatic Brain Injury. In S. Honeybul & A. G. Kolias (Eds.), Traumatic Brain Injury. Springer. https://link.springer.com/chapter/10.1007/978-3-030-78075-3_1
- ↑ Papa, L., Brophy, G. M., Welch, R. D., Lewis, L. M., Braga, C. F., Tan, C. N., Ameli, N. J., Lopez, M. A., Haeussler, C. A., Mendez Giordano, D. I., Silvestri, S., Giordano, P., Weber, K. D., Hill-Pryor, C., & Hack, D. C. (2016). Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurology, 73(5), 551. https://doi.org/10.1001/jamaneurol.2016.0039
- ↑ Rose, F. C. (1997). The history of head injuries: An overview*. Journal of the History of the Neurosciences, 6(2), 154–180. https://doi.org/10.1080/09647049709525700
- ↑ Sanchez, G. M., & Burridge, A. L. (2007). Decision making in head injury management in the Edwin Smith Papyrus. Neurosurgical Focus, 23(1), 1–9. https://doi.org/10.3171/foc-07/07/e5
- ↑ The History of Brain Injuries. (2018, March 29). Intrepid Fallen Heroes Fund. Retrieved October 17, 2022, from https://www.fallenheroesfund.org/the-history-of-brain-injuries
- ↑ Feinsod, M. (1997). Three head injuries: The biblical account of the deaths of Sisera, Abimelech and Goliath. Journal of the History of the Neurosciences, 6(3), 320–324. https://doi.org/10.1080/09647049709525717
- ↑ Hippocrates. (n.d.). On Injuries of the Head by Hippocrates (F. Adams, Trans.). The Internet Classics Archive. Retrieved October 17, 2022, from http://classics.mit.edu/Hippocrates/headinjur.13.13.html
- ↑ Ikram, M. Q., Sajjad, F. H., & Salardini, A. (2016). The head that wears the crown: Henry VIII and traumatic brain injury. Journal of Clinical Neuroscience, 28, 16–19. https://doi.org/10.1016/j.jocn.2015.10.035
- ↑ Marshall, L. F. (2000). Head Injury: Recent Past, Present, and Future. Neurosurgery, 47(3), 546–561. https://doi.org/10.1097/00006123-200009000-00002
- ↑ Giordano, K. R., & Lifshitz, J. (2021). Pathophysiology of Traumatic Brain Injury. In S. Honeybul & A. G. Kolias (Eds.), Traumatic Brain Injury. Springer. https://doi.org/10.1007/978-3-030-78075-3_2
- ↑ https://qbi.uq.edu.au/brain-basics/brain/brain-physiology/types-glia
- ↑ Sofroniew, M. V., & Vinters, H. V. (2009). Astrocytes: biology and pathology. Acta Neuropathologica, 119(1), 7–35. https://doi.org/10.1007/s00401-009-0619-8
- ↑ Verkhratsky, A., & Butt, A. (2013). General Pathophysiology of Neuroglia. In Glial Physiology and Pathophysiology (1st ed.). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781118402061
- ↑ Verkhratsky, A., & Butt, A. (2013). General Pathophysiology of Neuroglia. In Glial Physiology and Pathophysiology (1st ed.). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781118402061
- ↑ Messing, A., & Brenner, M. (2020). GFAP at 50. ASN Neuro, 12, 175909142094968. https://doi.org/10.1177/1759091420949680
- ↑ Abdelhak, A., Foschi, M., Abu-Rumeileh, S., Yue, J. K., D’Anna, L., Huss, A., Oeckl, P., Ludolph, A. C., Kuhle, J., Petzold, A., Manley, G. T., Green, A. J., Otto, M., & Tumani, H. (2022). Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nature Reviews Neurology, 18(3), 158–172. https://doi.org/10.1038/s41582-021-00616-3
- ↑ Dewan, M. C., Rattani, A., Gupta, S., Baticulon, R. E., Hung, Y. C., Punchak, M., Agrawal, A., Adeleye, A. O., Shrime, M. G., Rubiano, A. M., Rosenfeld, J. V., & Park, K. B. (2019). Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery, 130(4), 1080–1097. https://doi.org/10.3171/2017.10.jns17352
- ↑ James, S. L., Theadom, A., Ellenbogen, R. G., Bannick, M. S., Montjoy-Venning, W., Lucchesi, L. R., Abbasi, N., Abdulkader, R., Abraha, H. N., Adsuar, J. C., Afarideh, M., Agrawal, S., Ahmadi, A., Ahmed, M. B., Aichour, A. N., Aichour, I., Aichour, M. T. E., Akinyemi, R. O., Akseer, N., . . . Murray, C. J. L. (2019). Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology, 18(1), 56–87. https://doi.org/10.1016/s1474-4422(18)30415-0
- ↑ Georges, A., & Das, J. M. (2022). Traumatic Brain Injury [Internet]. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459300/
- ↑ Seel, R. T., Macciocchi, S., & Kreutzer, J. S. (2010). Clinical Considerations for the Diagnosis of Major Depression After Moderate to Severe TBI. Journal of Head Trauma Rehabilitation, 25(2), 99–112. https://doi.org/10.1097/htr.0b013e3181ce3966
- ↑ https://smhs.gwu.edu/urgentmatters/news/keep-it-simple-acute-gcs-score-binary-decision
- ↑ Riechers, R. G., Ramage, A., Brown, W., Kalehua, A., Rhee, P., Ecklund, J. M., & Ling, G. S. (2005). Physician Knowledge of the Glasgow Coma Scale. Journal of Neurotrauma, 22(11), 1327–1334. https://doi.org/10.1089/neu.2005.22.1327
- ↑ Institute of Medicine (US) Committee on Gulf War and Health: Brain Injury in Veterans and Long-Term Health Outcomes. (2008). Gulf War and Health: Volume 7 (Vol. 7) [Internet]. National Academies Press. https://doi.org/10.17226/12436
- ↑ Cho, D. Y., & Wang, Y. C. (1997). Comparison of the APACHE III, APACHE II and Glasgow Coma Scale in acute head injury for prediction of mortality and functional outcome. Intensive Care Medicine, 23(1), 77–84. https://doi.org/10.1007/s001340050294
- ↑ McKinlay, A., Lin, A., & Than, M. (2018). A comparison of emergency department medical records to parental self-reporting of traumatic brain injury symptoms. Concussion, 3(1), CNC52. https://doi.org/10.2217/cnc-2017-0017